Answer:
6 chloride ions surround each lithium ion in LiCl unit cell.
Step-by-step explanation:
Lithium chloride crystallizes into face-centered cubic unit cell.
Face-centered cubic structures are type of cubic close packing.
Number of octahedral voids in ccp = n
Number of tetrahedral voids in ccp = 2n
n = number of atoms or ions in the packing
In FCC , crystal lattice there are 4 atoms.
Number of octahedral voids in FCC= 4
1 octahdreal void is present at center of the cube and 12 octahedral voids are present at the center of 12 edges of the cube. Each edge of a cube is shared by 4 unit cells.
1+
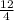 = 4
= 4
In unit cell of LiCl, lithium is present at octahedral void. And each octahedral void is surrounded by 6 chlorine atoms.
As chlorine ions present at all the faces of the cube are touching lithium ion present in an octahedral void.