Answer:
The amount of heat associated with 169 g of mercury is 1.557 kiloJoules.
Step-by-step explanation:
Mass of mercury = m = 169 g
Change in temperature of the mercury =
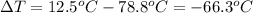
The specific heat of mercury = c = 0.139 J/g°C
Heat associated with mercury: Q
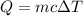
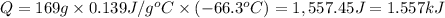
Negative sign indicates that heat was released when mercury is cooled down.
The amount of heat associated with 169 g of mercury is 1.557 kiloJoules.