Step-by-step explanation:
All the atoms present in periodic table are neutral in nature and it is known that a neutral atom means that it contains no charge.
For example, sodium is present as Na in the periodic table. And, it does not contain any charge.
When a neutral atom loses or gains an electron then it acquires a charge.
For example, atomic number of calcium is 20 and its electronic distribution is 2, 8, 8, 2. Hence, in order to acquire stability when it loses 2 valence electrons then neutral atom of Ca changes into
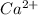 .
.
Hence, it means that by loosing 2 valence electrons calcium has an electric charge of +2.