Step-by-step explanation:
As the given equations are as follows.
Step 1:
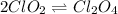 (fast)
(fast)
Step 2:
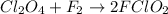 (slow)
(slow)
So, in order to determine the overall reaction we will add both step 1 and step 2 as follows.
Step 1 + 2 :
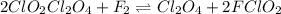
Thus, by cancelling the common species we can conclude that the equation for overall reaction is
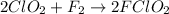 .
.