Step-by-step explanation:
The given reaction will be as follows.
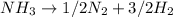
As all the ammonia has decomposed into nitrogen and hydrogen. Hence, there will be no ammonia left in the reaction.
Now, using Dalton's law, partial pressure of
 + partial pressure of
+ partial pressure of
 = 821 mm Hg.
= 821 mm Hg.
As there is 1/2 mole
 for 3/2 moles
for 3/2 moles
 , the partial pressure of
, the partial pressure of
 is 1/3 the partial pressure of the
is 1/3 the partial pressure of the
 .
.
Let the partial pressure of
 will be x and partial pressure of
will be x and partial pressure of
 is therefore 3x.
is therefore 3x.
Hence, we will calculate the value of x as follows.
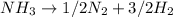
x + 3x = 821 mm Hg
4x = 821 mm Hg
x = 205.25
Therefore, we can conclude that the partial pressure of
 is 205.25 mm Hg, partial pressure of
is 205.25 mm Hg, partial pressure of
 is 615.75 mm Hg.
is 615.75 mm Hg.