Answer: Odor of ammonia would we detect first on the other side of the room.
Step-by-step explanation:
To calculate the rate of diffusion of gas, we use Graham's Law.
This law states that the rate of effusion or diffusion of gas is inversely proportional to the square root of the molar mass of the gas. The equation given by this law follows:
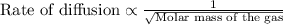
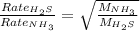
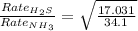
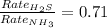
Thus the odor of ammonia would we detect first on the other side of the room as the rate of effusion of ammonia would be faster as it has low molecular weight as compared to hydrogen sulphide.