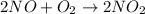Step-by-step explanation:
As the given reactions are as follows.
Step 1:
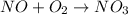 (fast)
(fast)
Step 2:
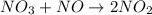 (slow)
(slow)
As it is known that a reaction that occurs slowly helps in determining the rate of a reaction.
So, to find overall reaction we will add both step 1 and step 2 by cancelling out common species as follows.
Step 1 + 2 =
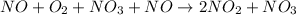
Hence, by cancelling the common species the overall reaction is as follows.