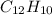Answer:
The molecular formula of an ascenapthalene is
Step-by-step explanation:
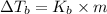
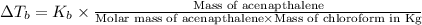
where,
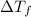 =Elevation in boiling point =
=Elevation in boiling point =
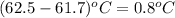
Mass of acenapthalene = 0.515 g
Mass of
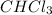 = 15.0 g = 0.015 kg (1 kg = 1000 g)
= 15.0 g = 0.015 kg (1 kg = 1000 g)
 = boiling point constant = 3.63 °C/m
= boiling point constant = 3.63 °C/m
m = molality
Now put all the given values in this formula, we get
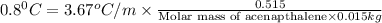
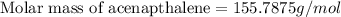
Let the molecule formula of the Acenapthalene be
![C_{6n]H_(5n)](https://img.qammunity.org/2020/formulas/chemistry/high-school/b96xu15kqlzsk400mf2gbk0talprhr6esl.png)
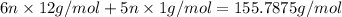
n = 2.0
The molecular formula of an ascenapthalene is