Answer:
39.8 °C
Step-by-step explanation:
m = mass of the ice = 100 g
L = latent heat of fusion of ice = 334 Jg⁻¹
M = mass of water = 200 g
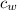 = specific heat of water = 4.2 Jg⁻¹°C⁻¹
= specific heat of water = 4.2 Jg⁻¹°C⁻¹
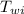 = initial temperature of water = ?
= initial temperature of water = ?
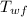 = final temperature of water = 0 °C
= final temperature of water = 0 °C
Using conservation of heat
Heat gained by ice = heat lost by water
m L = M
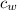 (
(
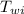 -
-
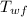 )
)
(100) (334) = (200) (4.2) (
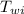 - 0 )
- 0 )
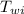 = 39.8 °C
= 39.8 °C