Step-by-step explanation:
As it is known that relation between heat and specific heat is as follows.
Q =
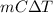
Heat evolved during phase change for temperature
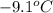 to
to
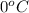 is as follows.
is as follows.
Q =
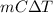
=
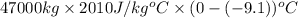
= 859677000 J ............ (1)
Heat evolved during phase change for temperature
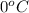 to
to
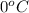 is as follows.
is as follows.
Q =
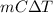
As temperature remains constant so, heat released will be equal to Q = m × l. Where l is latent heat of fusion on water equals 334 J/g.
Q = m × l
=
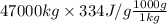
= 15698000000 J ........... (2)
Heat evolved during phase change for temperature
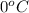 to
to
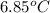 is as follows.
is as follows.
Q =
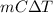
=
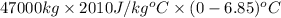
= 647119500 J .......... (3)
Now, total heat will be the sum of equations (1) + (2) + (3) as follows.
859677000 J + 15698000000 J + 647119500 J
= 17204796500 J
Also, relation between entropy and heat is as follows.
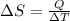
=
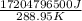
= 59542469.28 J/K
Thus, we can conclude that final change in entropy is 59542469.28 J/K.