Answer : The rate order of
 is, 1
is, 1
Explanation :
The given balanced reaction is,
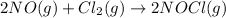
As we know that rate is an experimentally determined value. So, we can not determine the rate law by looking at the reaction.
The general rate law expression will be,
![R=k[NO]^x[Cl_2]^y](https://img.qammunity.org/2020/formulas/chemistry/college/mm0wd2sy2a1kl0cpfd6h8nvx6b2fcfgxse.png)
where,
R = rate
k = rate constant
![[NO]](https://img.qammunity.org/2020/formulas/chemistry/college/2n3pvq040it0tve0k7qo6ka6ls9r6hu9v3.png) and
and
![[Cl_2]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/v75bkiprjls8kiamt8nz609kyhnons8726.png) = concentration of NO and
= concentration of NO and
 reactant
reactant
x and y are the order of the reaction of NO and
 reactant respectively.
reactant respectively.
To calculate the order with respect to
 , we can compare the rates of two reactions in which the concentration of NO remains the same.
, we can compare the rates of two reactions in which the concentration of NO remains the same.
Now we have to compare the rates 2 and 3 experiment because in this NO concentration remains same and we get:
![(R_2)/(R_3)=(k[NO]^x[Cl_2]^y)/(k[NO]^x[Cl_2]^y)](https://img.qammunity.org/2020/formulas/chemistry/college/xer2viaio9d55owt1yzvq9cx77sur6jr6z.png)
![(0.08)/(0.16)=(k[0.8]^x[0.3]^y)/(k[0.8]^x[0.6]^y)](https://img.qammunity.org/2020/formulas/chemistry/college/35e44uf021i5x37te0zsqfzik8kgpicu0p.png)
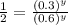
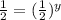
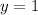
Therefore, the rate order of
 is, 1
is, 1