Answer:
Step-by-step explanation:
Polyatomic ions are ions that contain more than one atom in each one of the ions.
Ionic compounds are made of cations (positive ions) and anions (negative ion). By convention, the cation is written before the anion.
List the ions in each compound:
CaCO₃
Cation:
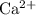 ,
,
Anion:
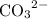 .
.
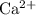 is a monatomic ion for it contains only one atom in each ion.
is a monatomic ion for it contains only one atom in each ion.
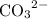 is a polyatomic ion for it contains four atoms (one C atom and three O atoms) in each ion.
is a polyatomic ion for it contains four atoms (one C atom and three O atoms) in each ion.
NaF
Cation:
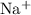 ,
,
Anion:
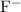 .
.
Both
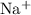 and
and
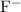 are monatomic for they contain only one atom in each ion.
are monatomic for they contain only one atom in each ion.
FeO
Cation:
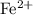 ,
,
Anion:
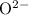 .
.
Both
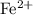 and
and
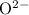 are monatomic for they contain only one atom in each ion.
are monatomic for they contain only one atom in each ion.
CaCl₂
Cation:
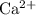 ,
,
Anion:
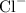 .
.
Both
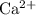 and
and
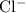 are monatomic for they contain only one atom in each ion.
are monatomic for they contain only one atom in each ion.
NaOH
Cation:
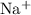 ,
,
Anion:
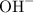 .
.
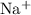 is a monatomic ion for it contains only one atom in each ion.
is a monatomic ion for it contains only one atom in each ion.
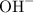 is a polyatomic ion for it contains two atoms (one O atom and one H atom) in each ion.
is a polyatomic ion for it contains two atoms (one O atom and one H atom) in each ion.