Answer: Option (d) is the correct answer.
Step-by-step explanation:
A covalent bond is formed when sharing of electrons take place between the combining atoms.
For example, in a
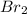 sharing of one electron takes place between both the bromine atoms.
sharing of one electron takes place between both the bromine atoms.
Whereas if a chemical bond is formed by transfer of electrons from one atom to another then it is called an ionic bond.
For example, sodium has one valence electron and it will readily donate its one electron to another atom in order to attain stability.
As a result, it will always form an ionic bond.
Thus, we can conclude that if an atom has one valence electron—that is, a single electron in its outer energy level—it will most likely form an ionic bond.