Answer: 212 g of sodium carbonate must react
Step-by-step explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products formed must be equal to the mass of reactants taken.
In order to get the same mass on both sides, the atoms of each element must be balanced on both sides of the chemical equation.
The balanced chemical equation is:
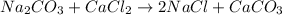
Mass of products = mass of sodium chloride + mass of calcium carbonate = 233 g+ 201 g= 434 g
Mass of reactants= mass of sodium carbonate + mass of calcium chloride =x + 222 g
As the mass of reactants should be equal to mass of products
434 = x + 222 g
x = 212 g
Thus 212 g of sodium carbonate must react with 222 g of calcium chloride to make 233 grams of sodium chloride and 201 grams of calcium carbonate