Answer:
The molecular weight of
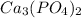 is 310.18 g/mol.
is 310.18 g/mol.
Step-by-step explanation:
The molecular weight of
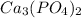 :
:
Atomic mass of calcium = 40.08 g/mol
Atomic mass of phosphorous = 30.97 g/mol
Atomic mass of oxygen = 16.00 g/mol
Number of calcium atom = 3
Number of phosphorus atom =1 × 2
Number of oxygen atom =4 × 2 = 8
Molecular weight of
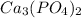 = M
= M
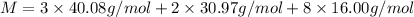
M = 310.18 g/mol