Answer:
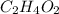 is the Molecular formula.
is the Molecular formula.
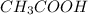 is the Chemical formula and its name is acetic acid or Ethanoic acid.
is the Chemical formula and its name is acetic acid or Ethanoic acid.
The number goes as subscript in the formula.
To find the number of atoms of each element we multiply coefficient × subscript
For example
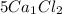 contains
contains
5 × 1 = 5 , Ca atoms and
5 × 2 = 10, Cl atoms
If there is a bracket in the chemical formula
For example
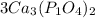 we multiply coefficient × subscript × number outside the bracket.......... to find the number of atoms (Please note: 3 is the coefficient, and if there is no number given then 1 will be the coefficient )
we multiply coefficient × subscript × number outside the bracket.......... to find the number of atoms (Please note: 3 is the coefficient, and if there is no number given then 1 will be the coefficient )
So 3 × 3 = 9, Ca atoms
3 × 1 × 2 = 6, P atoms
3 × 4 × 2 = 24, O atoms are present.