Answer:
197.4 atm is the Answer.
Step-by-step explanation:
Pressure can be expressed in unit of torr, mm Hg, psi, atm, kPa etc
1 atm = 760 torr = 760 mm Hg = 14.7 psi = 101.325 kPa
So Here to convert atm to kPa the conversion factor is either
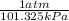 or
or
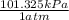
We need the answer in atm so kPa should get cancel and it should be in the denominator
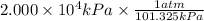
=197.4 atm is the Answer.