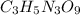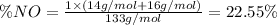Answer:
Mass percentage of NO in nitroglycerin is 39.63%.
Mass percent of NO in isoamyl nitrate is 22.55\%.
Step-by-step explanation:
Molar mas of nitroglycerin ,
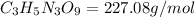
Molar mas of isoamyl nitrate,
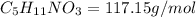
Given: If each compound releases one molecule of NO per atom of N.
1)In nitroglycerin, there are 3 nitrogen atoms. then number of NO will be :
3 × 1= 3 NO molecules
Mass percent of NO in
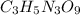
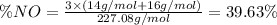
2)In isoamyl nitrate. , there are 1 nitrogen atom. then number of NO will be :
1 × 1= 3 NO molecules
Mass percent of NO in