Answer:
No, there are not enough Ba atoms
Step-by-step explanation:
Mass of
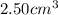 of Ba =
of Ba =
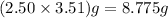
Mass of
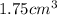 of S =
of S =
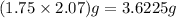
Molar mass of Ba = 137.33 g
Molar mass of S = 32.06 g
1 mol of an element contains
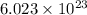 number of atoms.
number of atoms.
So 8.775 g of Ba =
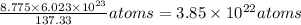
So 3.6225 g of S =
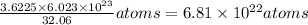
As 1 atom of Ba reacts with 1 atom of S therefore enough Ba atoms are not present for reaction.