Step-by-step explanation:
(a) The given data is as follows.
Concentration = 1 mg/L, Toxic amount = 0.2 g
Now, we will calculate the volume of fluoridated drinking water as follows.
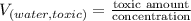
=
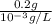
= 200 L
Hence, there will be 200 liters of fluoridated drinking water would a 70-kg person have to consume in one day to reach this toxic level.
(b) Now, it is also given that
V =
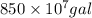
We will convert gallons into liters as follows.
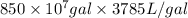
=
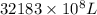
Concentration = 1 mg/L
Therefore, we will calculate the mass as follows.
m =

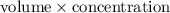
=
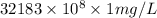
=
 (As 1 mg =
(As 1 mg =
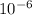 kg)
kg)
Thus, we can conclude that there are
 of sodium fluoride would be needed to treat a
of sodium fluoride would be needed to treat a
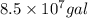 reservoir.
reservoir.