Answer:
Here's what I get
Step-by-step explanation:
(a) Mass % of each ion
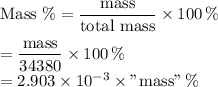
I used this template to calculate the percentages in the table below.
Ion Abundance Mass %
Na⁺ 10 560 30.72
K⁺ 380 1.11
Alkali metals 10 940 31.82
Mg²⁺ 1 270 3.69
Ca²⁺ 400 1.16
Alkaline earth metals 1 670 4.86
Total metal ions 12 610 36.68
Cl⁻ 18 980 55.20
SO₄²⁻ 2 650 7.71
HCO₃⁻ 140 0.41
Anions 21 770 63.32
TOTAL 34 380 100.00
(b) 30.72 % of the total mass is sodium ion.
(c) Alkaline earth metals vs alkali metals
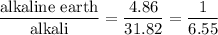
The mass percent of alkali metal ions is 6.55 times that of alkaline earth metal ions.
(d) The mass of anions is greater than that of cations.