Answer:
For a: The number of oxygen atoms in given compound is
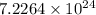 and molecular mass of the compound is 342 g.
and molecular mass of the compound is 342 g.
For b: The number of hydrogen atoms in given compound is
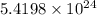 and molecular mass of the compound is 132 g.
and molecular mass of the compound is 132 g.
For c: The number of hydrogen atoms in given compound is
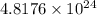 and molecular mass of the compound is 344.5 g
and molecular mass of the compound is 344.5 g
Step-by-step explanation:
Formula units is defined as lowest whole number ratio of ions in an ionic compound. It is calculate by multiplying the number of moles by Avogadro's number which is

Molecular mass is defined as the sum of atomic weights of all the atoms present in the molecular formula.
We are given a chemical compound having formula of
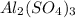
In 1 mole of aluminium sulfate, 2 moles of aluminium atoms, 3 moles of sulfur atoms and 12 moles of oxygen atoms are present.
So, number of atoms of oxygen in 1 formula unit of the compound =
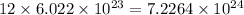
Molecular mass of aluminium sulfate =
![[(27* 2)+3(32+(4* 16))]=342g](https://img.qammunity.org/2020/formulas/chemistry/college/ch4yu7qn8td48lhno5xhgbw0dkvblvggy8.png)
Hence, the number of oxygen atoms in given compound is
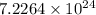 and molecular mass of the compound is 342 g.
and molecular mass of the compound is 342 g.
We are given a chemical compound having formula of
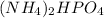
In 1 mole of ammonium hydrogen phosphate, 2 moles of nitrogen atoms, 9 moles of hydrogen atoms, 1 mole of phosphorus atom and 4 moles of oxygen atoms are present.
So, number of atoms of hydrogen in 1 formula unit of the compound =
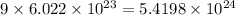
Molecular mass of ammonium hydrogen phosphate =
![[2(14+(4* 1)+(1+31+(4* 16))]=132g](https://img.qammunity.org/2020/formulas/chemistry/college/ptcyf4ikiawepfdgvo4v9tux3qhgjv4ktx.png)
Hence, the number of hydrogen atoms in given compound is
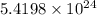 and molecular mass of the compound is 132 g.
and molecular mass of the compound is 132 g.
We are given a chemical compound having formula of
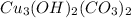
In 1 mole of azurite, 3 moles of copper atoms, 2 moles of hydrogen atoms, 2 mole of carbon atom and 8 moles of oxygen atoms are present.
So, number of atoms of oxygen in 1 formula unit of the compound =
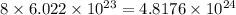
Molecular mass of azurite =
![[(3* 63.5)+2(16+1)+2(12+(3* 16))]=344.5g](https://img.qammunity.org/2020/formulas/chemistry/college/t6sxqbrtr0iodionitoza9gtnowfotxxyt.png)
Hence, the number of hydrogen atoms in given compound is
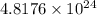 and molecular mass of the compound is 344.5 g
and molecular mass of the compound is 344.5 g