Answer:
a. Copper (I)Iodide.
b.Iron (III)Hydrogen sulfate.
c. Magnesium Chromate.
Step-by-step explanation:
a.Cu I
Copper(I) Iodide
We know that charge on iodide ion is -1 and charge on +1.
Hence, the correct name is copper (I)Iodide.
b.
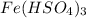
We know that charge on hydrogen sulfate ion is -1
and charge on iron is +3
Then , it require 3 ion of hydrogen sulfate to form iron Hydrogen sulfate.
Hence, the correct name is Iron (III)hydrogen sulfate.
c.
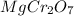
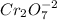 is Chromate ion
is Chromate ion
The charge on magnesium is +2.
Hence, the correct name is Magnesium Chromate.