Answer: ionic compounds
Step-by-step explanation:
The nomenclature of covalent compound is given by:
1. The less electronegative element is written first.
2. The more electronegative element is written then, and a suffix is added with it. The suffix added is '-ide'.
3. If atoms of an element is greater than 1, then prefixes are added which are 'mono' for 1 atom, 'di' for 2 atoms, 'tri' for 3 atoms and so on..
For example: IUPAC name for
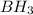 is boron trihydride.
is boron trihydride.
The nomenclature of ionic compounds is given by:
1. Positive is written first followed by the oxidation state of metal roman numerals in square brackets.
2. The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
Hence, the name of
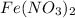 is iron (II) nitrate.
is iron (II) nitrate.