Step-by-step explanation:
The given reaction will be as follows.
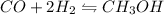
So, equilibrium constant for this equation will be as follows.
![K_(c) = ([CH_(3)OH])/([CO][H_(2)]^(2))](https://img.qammunity.org/2020/formulas/chemistry/high-school/pwucqm634tan5fjhbh3l8vxwmq8yioj2u6.png)
As it is given that concentration of all the species is 2.4. Therefore, calculate the value of equilibrium constant as follows.
![K_(c) = ([CH_(3)OH])/([CO][H_(2)]^(2))](https://img.qammunity.org/2020/formulas/chemistry/high-school/pwucqm634tan5fjhbh3l8vxwmq8yioj2u6.png)
=
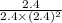
= 0.173
Thus, we can conclude that equilibrium constant for the given reaction is 0.173.