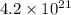Answer: The number of oxide ions that must be present is
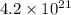
Step-by-step explanation:
Lithium is the 3rd element of the periodic table having electronic configuration of
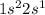
This element will loose 1 electron to form
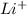 ion.
ion.
Oxygen is the 8th element of the periodic table having electronic configuration of
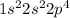
This element will gain 2 electrons to form
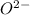 ion.
ion.
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
So, the chemical formula of compound is
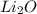
According to mole concept:
1 mole of an ionic compound contains
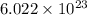 number of ions.
number of ions.
In the given ionic compound, the number of lithium ions present are
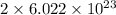 and number of oxide ions present are
and number of oxide ions present are
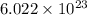
So, in the given ionic compound:
If
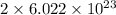 number of lithium ions are present, then
number of lithium ions are present, then
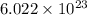 number of oxide ions will be present.
number of oxide ions will be present.
So,
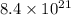 number of lithium ions are present, then
number of lithium ions are present, then
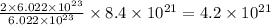 number of oxide ions will be present.
number of oxide ions will be present.
Hence, the number of oxide ions that must be present is