Answer: The correct answer is D.
Step-by-step explanation:
We are given two reactions which are the two steps of a mechanism:
![A+B\xrightarrow[]{slow}C+D](https://img.qammunity.org/2020/formulas/chemistry/high-school/qxyp0fv5t95aektymcd1eniial07i5jxk1.png) ......(1)
......(1)
![A+C\xrightarrow[]{fast}E+F](https://img.qammunity.org/2020/formulas/chemistry/high-school/53x9vm9ef40m7g9pnr8wyu3qya1y4l08a9.png) .......(2)
.......(2)
To determine the net chemical equation, we will simply add the above two equations, we get:
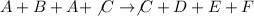
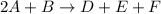
The rate of the reaction depends on the slow step of the mechanism, which is:
![Rate=k[A][B]](https://img.qammunity.org/2020/formulas/chemistry/high-school/uevpc2zc21j9lphsqvh6kap3tdimfnefmb.png)
Thus, the correct answer is Option D.