Answer: Group 1 ions are known as cations and Group 17 ions are known as anions.
Step-by-step explanation:
Ions are formed when an atom looses or gains electrons.
If an atom gains electrons, it leads to the formation of negative ions known as anions. For Example: Fluorine is a Group 17 element which gains 1 electron to form
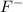 ions.
ions.
If an atom looses electrons, it leads to the formation of positive ions known as cations. For Example: Sodium is a Group 1 element which looses 1 electron to form
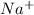 ions.
ions.
Hence, group 1 ions are known as cations and Group 17 ions are known as anions.