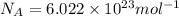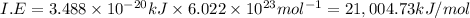Answer:
The ionization energy (in kJ/mol) of the helium ion is 21,004.73 kJ/mol .
Step-by-step explanation:
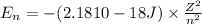
Z = atomic mass
n = principal quantum number
Energy of the electron in n=1,
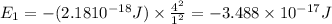
Energy of the electron in n = ∞
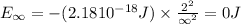
Ionization energy of the
 ion:
ion:
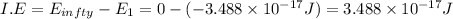
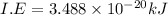
To convert in into kj/mol multiply it with