Answer : The mass of a sample of water is, 888.89 grams
Explanation :
Latent heat of vaporization : It is defined as the amount of heat energy released or absorbed when the liquid converted to vapor at atmospheric pressure at its boiling point.
Formula used :
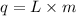
where,
q = heat = 2000 kJ =
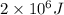 (1 kJ = 1000 J)
(1 kJ = 1000 J)
L = latent heat of vaporization of water =
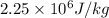
m = mass of sample of water = ?
Now put all the given values in the above formula, we get:
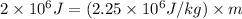
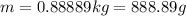 (1 kg = 1000 g)
(1 kg = 1000 g)
Therefore, the mass of a sample of water is, 888.89 grams