Answer:
Compound A is 2-bromo-3-ethylpentane. Compound B is 3-ethylpent-2-ene. Compound C is 3-ethylpent-1-ene. Structures are given below.
Step-by-step explanation:
Sodium ethoxide in ethanol gives E2 ellimination by removing HBr when it is treated with 2-bromo-3-ethylpentane.
2-bromo-3-ethylpentane (
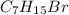 has two possible positions to give ellimination products.
has two possible positions to give ellimination products.
3-ethylpent-2-ene is the major product as it contains more substituted double bond and 3-ethylpent-1-ene is the minor product as it contains less substituted double bond.
Both 3-ethylpent-2-ene and 3-ethylpent-1-ene gives only one product due to catalytic hydrogenation through addition of
 in double bond.
in double bond.
Structures are given below.