Answer:
emission of photon is of maximum wavelength when electron transition is from n = 8 to n = 5
Step-by-step explanation:
Longest wavelength for the transition means the energy difference in two levels must me minimum
So here we know that the energy of electron in a given level is
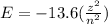
now if z = 1 for hydrogen
then Energy of electron for n = 2
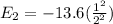
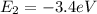
Energy of electron for n = 5
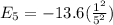
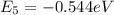
Energy of electron for n = 8
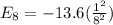
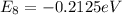
Now for least energy difference we can say
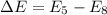
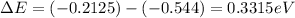
So emission of photon is of maximum wavelength when electron transition is from n = 8 to n = 5