Answer:
Step-by-step explanation:
Given parameters:
Inital volume of argon, V = 5m³
Initial temperature of argon, T = 425K
Initial pressure of argon, P = 3.9atm
Final temperature when cooled T₂ = 240K
Final volume when cooled V₂ = 3.1m³
Pressure of the helium gas = 1.87atm
Uknown parameters:
Total mixture of the gas = Ptotal
Solution
To find the total pressure of the gas mixture, we must determined the final pressure of the cooled Ar gas. Then the total pressure can be obtained using the expression below:
Ptotal = P₁ + P₂
Where P₁ and P₂ are the partial pressure exterted by Ar and He in the mixture.
Using the general gas law, we find the final pressure exterted when the argon gas cooled:
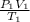 =
=
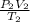
The unkown here is P₂ and simply make it the subject of the expression above:
P₂ =
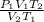
P₂ =
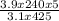 = 3.55atm
= 3.55atm
Partial pressure exerted by Ar is 3.55atm
Ptotal = 3.55atm + 1.87atm = 5.42atm