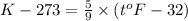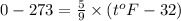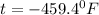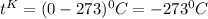Answer:
a)
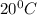 and
and
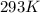
b)
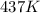 and
and
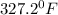
c)
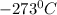 and
and
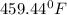
Step-by-step explanation:
Temperature of the gas is defined as the degree of hotness or coldness of a body. It is expressed in units like
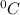 ,
,
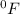 and
and
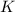
These units of temperature are inter convertible.
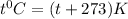
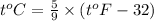
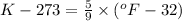
a) 68°F (a pleasant spring day) to °C and K.
Converting this unit of temperature into
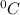 and
and
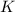 by using conversion factor:
by using conversion factor:
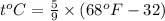
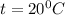
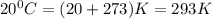
b) 164°C (the boiling point of methane, the main component of natural gas) to K and °F
Conversion from degree Celsius to Kelvins and Fahrenheit
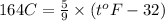
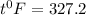
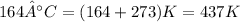
c) 0K (absolute zero, theoretically the coldest possible temperature) to °C and °F.