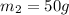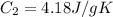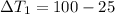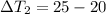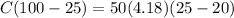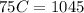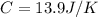Answer:
C = 13.9 J/K
Step-by-step explanation:
Here we are given that no energy is lost in the surrounding so we can say that there energy is conserved in this system
So we will have
energy given by hot piece of copper = energy absorbed by the coffee
So we will have
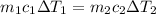
so we will have