Answer:
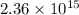 metric tons of oxygen is present.
metric tons of oxygen is present.
Step-by-step explanation:
We are given:
Mass of oxygen in atmosphere =
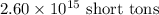
To convert it into metric tons, we use the conversion factors:
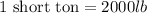
And,
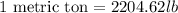
Converting short tons into pounds, we get:
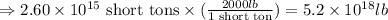
Now, converting the given mass in pounds to metric tons, we get:
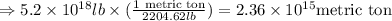
Hence,
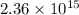 metric tons of oxygen is present.
metric tons of oxygen is present.