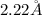Answer : The radius of a barium atom in angstroms is,
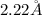
Explanation :
The conversion used for radius from meter to angstroms is:
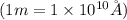
As we are given the radius of a barium atom in meter (m) is,
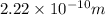
Now we have to determine the radius of a barium atom in angstrom.
As,
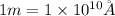
So,
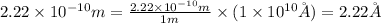
Therefore, the radius of a barium atom in angstroms is,