Answer:
The volume of the olive oil is 0.468 L.
Step-by-step explanation:
Given that,
Mass of lead m₁= 2.07 kg
Initial temperature of lead = 92.0°C
Initial temperature of oil = 41.0°C
Density of olive oil
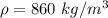
Specific heat of lead
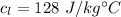
Specific heat of olive oil
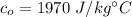
We need to calculate the mass of the olive oil
The heat lost by the lead equal to the heat gained by olive oil
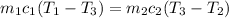
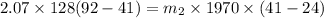
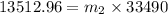
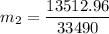
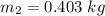
We need to calculate the volume of olive oil
Using formula of density of olive oil
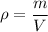
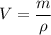
Put the value into the formula
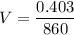
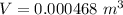
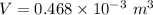
Hence, The volume of the olive oil is 0.468 L.