Answer:
Net ionic equation:
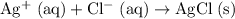 .
.
Step-by-step explanation:
Start by balancing the chemical equation. The acetate (IUPAC: ethanoate) ion
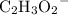 is a polyatomic ion. These ions stayed intact during this reaction. These ions could thus be considered as a monatomic ion
is a polyatomic ion. These ions stayed intact during this reaction. These ions could thus be considered as a monatomic ion
 to make this equation easier to balance.
to make this equation easier to balance.
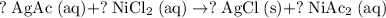
Assign a coefficient of 1 to
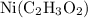 :
:
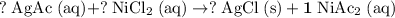
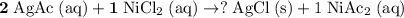
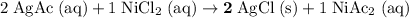
Rewrite this chemical equation as an ionic equation. For each salt in this reaction: if the salt dissolves in water, rewrite it as the ions that in produce. Don't rewrite species that won't dissolve into ions.
For example:
- The salt
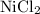 is soluble in water. It dissolves to produce
is soluble in water. It dissolves to produce
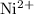 ions and
ions and
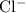 ions. Hence rewrite
ions. Hence rewrite
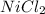 as
as
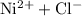
- The salt
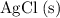 is insoluble in water. Do not rewrite.
is insoluble in water. Do not rewrite.
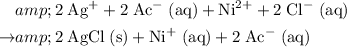 .
.
Eliminate terms that appear on both side of this equation.
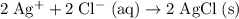 .
.
Simplify the coefficients:
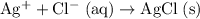 .
.