Answer: Enzymes enhances the rate in the same order.
Step-by-step explanation:
Catalyzed reactions are defined as the reactions in which a catalyst is used.
A catalyst is a substance which increases the rate of the reaction and does not participate in the reaction. It can be recovered at the end of the reaction. It decreases the activation energy of the reaction.
The rate of catalyzed reactions is always greater than the rate of uncatalyzed reactions.
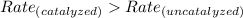
Enzymes are the natural catalysts which are present in the animal body to increase the rate of some reactions.
Thus, they enhance the rate of both slow and fast uncatalyzed reaction at the same rate.