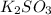Answer:
For a: The chemical formula for Iron (III) sulfide is
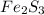
For b: The chemical formula for mercury (II) nitrate is
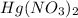
For c: The chemical formula for potassium sulfite is
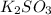
Step-by-step explanation:
All the given compounds are ionic compounds. This means that between the atoms complete sharing of electrons takes place.
Iron is the 26th element of periodic table having electronic configuration of
![[Ar]3d^64s^2](https://img.qammunity.org/2020/formulas/chemistry/college/wsovmfjor28tzhsix77vhy7ble1h6kn4h0.png) .
.
To form
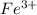 ion, this element will loose 3 electrons.
ion, this element will loose 3 electrons.
Sulfur is the 16th element of periodic table having electronic configuration of
![[Ne]3s^23p^4](https://img.qammunity.org/2020/formulas/chemistry/college/a9bazkdf9p6g2d58x8ogzse18oy4xqrw2a.png) .
.
To form
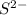 ion, this element will gain 2 electrons.
ion, this element will gain 2 electrons.
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
So, the chemical formula for Iron (III) sulfide is
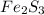
Mercury is the 80th element of periodic table having electronic configuration of
![[Xe]4f^(14)5d^(10)6s^2](https://img.qammunity.org/2020/formulas/chemistry/college/7011f4v0sou0rkkjm41xz8njaarkhbvjcr.png) .
.
To form
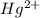 ion, this element will loose 2 electrons.
ion, this element will loose 2 electrons.
Nitrate ion is a polyatomic ion having chemical formula of
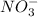
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
So, the chemical formula for mercury (II) nitrate is
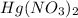
Potassium is the 19th element of periodic table having electronic configuration of
![[Ar]4s^1](https://img.qammunity.org/2020/formulas/chemistry/college/7kr4oi1hjrg0keyplq1ecucsnewl3ot0ah.png) .
.
To form
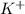 ion, this element will loose 1 electron.
ion, this element will loose 1 electron.
Sulfite ion is a polyatomic ion having chemical formula of
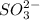
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
So, the chemical formula for potassium sulfite is