Answer :
(a) The name of
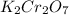 is, potassium dichromate.
is, potassium dichromate.
(b) The name of
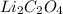 is, lithium oxalate.
is, lithium oxalate.
(c) The name of
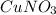 is, copper nitrate.
is, copper nitrate.
Step-by-step explanation:
Ionic compound : It is a type of compound that is made up of the ions of the two different elements in which one element is a metal and another element is a non-metal.
(a)
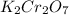
The given compound
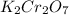 is an ionic compound. It is made up of two different ions which are potassium ion
is an ionic compound. It is made up of two different ions which are potassium ion
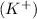 and dichromate ion
and dichromate ion
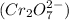 .
.
So, the name of
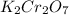 is, potassium dichromate.
is, potassium dichromate.
(b)
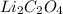
The given compound
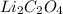 is an ionic compound. It is made up of two different ions which are lithium ion
is an ionic compound. It is made up of two different ions which are lithium ion
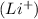 and oxalate ion
and oxalate ion
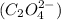 .
.
So, the name of
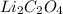 is, lithium oxalate.
is, lithium oxalate.
(c)
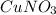
The given compound
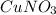 is an ionic compound. It is made up of two different ions which are copper ion
is an ionic compound. It is made up of two different ions which are copper ion
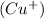 and nitrate ion
and nitrate ion
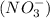 .
.
So, the name of
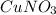 is, copper nitrate.
is, copper nitrate.