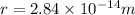Answer:
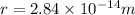
Step-by-step explanation:
As per energy conservation we know that the electrostatic potential energy of the charge system is equal to the initial kinetic energy of the alpha particle
So here we can write it as
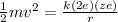
now we know that
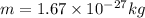

z = 79
here kinetic energy of the incident alpha particle is given as
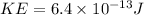
now we have
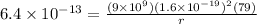
now we have