Answer: Option (a) is the correct answer.
Step-by-step explanation:
A chemical formula which represents proportions between the elements present in a compound is known as empirical formula.
When chemical or molecular formula is able to be reduced to smaller whole-number ratio of the elements then both molecular and empirical formulas are considered to be the same.
Relation between molecular formula and empirical formula is as follows.
Molecular formula = n × Empirical formula
where, n is an integer.
Chemical formula of acetic acid is
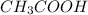 .
.
Chemical formula of formaldehyde is
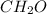 . It is the smaller whole-number ratio of molecular formula of acetic acid.
. It is the smaller whole-number ratio of molecular formula of acetic acid.
Chemical formula of Benzaldehyde is
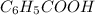 .
.
Chemical formula of glucose is
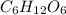 .
.
Thus, we can conclude that formaldehyde molecules has/have the same empirical formula as acetic acid (
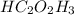 ).
).