Answer:
Manganese is the name and symbol of the element is Mn.
Step-by-step explanation:
Charge of the mono-atomic ion = 2+
Atomic mass of the nucleus = 55
Atomic mass =Number of protons + Number of neutrons
Given that :
Number of neutrons = 1.2 (Number of protons)
n = 1.2 × p
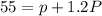
p = 25
Atomic number is equal to Number of total protons in the nucleus.
The atomic number of an element is 25 which Manganese with symbol 'Mn'.