Answer:
For a: The correct answer is
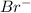
For b: The correct answer is Radon.
For c: The correct answer is
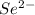
For d: The correct answer is
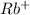
For e: The correct answer is
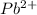
Step-by-step explanation:
An ion is formed when an atom looses or gains electron.
When an atom looses electrons, it will form a positive ion known as cation.
When an atom gains electrons, it will form a negative ion known as anion.
Atomic number is defined as the number of protons or electrons that are present in a neutral atom.
Atomic number = number of protons = number of electrons
Atomic mass is defined as the sum of number of protons and neutrons that are present in an atom.
Atomic mass = Number of protons + Number of neutrons
Halogens are Fluorine (Z = 9), Chlorine (Z = 17), Bromine (Z = 35), Iodine (Z = 53) and Astatine (Z = 85)
Bromine is the 35th element of the periodic table having electronic configuration
![[Ar]3d^(10)4s^24p^5](https://img.qammunity.org/2020/formulas/chemistry/college/9kgc8jkntqm5p6usyd213wabwdrwemvfs5.png)
This element will gain 1 electron and will form
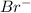 ion.
ion.
The ion formed has 36 electrons.
Noble gas having 86 protons means the element having atomic number 86 is Radon.
Thus, the correct answer is Radon.
Group 6A elements are Oxygen (Z = 8), Sulfur (Z = 16), Selenium (Z = 34), Tellurium (Z = 52) and Polonium (Z = 84)
Selenium is the 34th element of the periodic table having electronic configuration
![[Ar]3d^(10)4s^24p^4](https://img.qammunity.org/2020/formulas/chemistry/college/63lbjadcqsr8opo0qdrhle7ipj4gr0wts5.png)
This element will gain 2 electrons and will form
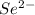 ion.
ion.
The ion formed has 36 electrons.
Alkali metals are Lithium (Z = 3), Sodium (Z = 11), Potassium (Z = 19), Rubidium (Z = 37), Cesium (Z = 55) and Francium (Z = 87)
Rubidium is the 34th element of the periodic table having electronic configuration
![[Kr]5s^1](https://img.qammunity.org/2020/formulas/chemistry/college/112z18rst6b8c66zzyzksvc6b7ohub6dyf.png)
This element will loose 1 electron and will form
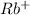 ion.
ion.
The ion formed has 36 electrons.
Group 4A elements are Carbon (Z = 6), Silicon (Z = 14), Germanium (Z = 32), Tin (Z = 50) and Lead (Z = 82)
Lead is the 82th element of the periodic table having electronic configuration
![[Xe]4f^(14)5d^(10)6s^26p^2](https://img.qammunity.org/2020/formulas/chemistry/college/wgy7o8ewuwxvgpcx7ybyuncuk4wfd379k7.png)
This element will loose 2 electrons and will form
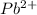 ion.
ion.
The ion formed has 80 electrons.