Answer: The given compounds obeys Law of multiple proportions.
Step-by-step explanation:
Law of multiple proportions states that when two elements combine to form two or more compounds in more than one proportion. The mass of one element that combine with a given mass of the other element are present in the ratios of small whole number. For Example:
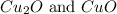
We are given:
Three compounds having chemical formulas of
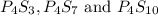
In the given compounds, mass ratio of phosphorus and sulfur are:
- Ratios of mass of elements in
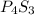
Mass of phosphorus = (4 × 31) = 124 g/mol
Mass of sulfur = (3 × 32) = 96 g/mol
Mass ratio of phosphorus and sulfur in
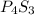 is 124 : 96
is 124 : 96
- Ratios of mass of elements in
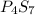
Mass of phosphorus = (4 × 31) = 124 g/mol
Mass of sulfur = (7 × 32) = 224 g/mol
Mass ratio of phosphorus and sulfur in
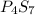 is 124 : 224
is 124 : 224
- Ratios of mass of elements in
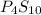
Mass of phosphorus = (4 × 31) = 124 g/mol
Mass of sulfur = (10 × 32) = 320 g/mol
Mass ratio of phosphorus and sulfur in
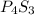 is 124 : 320
is 124 : 320
As, the mass of phosphorus is fixed, so the mass ratio of sulfur in both the compounds must be in whole number ratio.
Mass ratio of sulfur in both the compounds = 96 : 224 : 320 = 3 : 7 : 10
Hence, the given compounds obeys Law of multiple proportions.