Answer:
The complete equation (a) is:
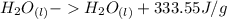 , and is an exothermica phase change
, and is an exothermica phase change
The complete equation (b) is:
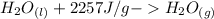 , and is an endothermic phase change.
, and is an endothermic phase change.
Step-by-step explanation:
Equation (a):
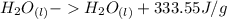 , is showing a phase change from a more-energetic state (liquid) to a relative-less-energetic state (solid), that means, that in order to have the phase change, it is requiered to remove heat (energy) from the water, this is known an exothermic (releases heat).
, is showing a phase change from a more-energetic state (liquid) to a relative-less-energetic state (solid), that means, that in order to have the phase change, it is requiered to remove heat (energy) from the water, this is known an exothermic (releases heat).
Equation (b).
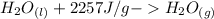 , shows that to change from a relative-less-energetic phase (liquid) to a more-energetic one (gas), it would be needed to supply energy in order to acomplish this. And it is called endothermic (absorves heat)
, shows that to change from a relative-less-energetic phase (liquid) to a more-energetic one (gas), it would be needed to supply energy in order to acomplish this. And it is called endothermic (absorves heat)