Answer:
A) Boyle's Law
Step-by-step explanation:
Boyle's Law states that pressure is inversely proportional to volume if the temperature and volume remain constant in closed system
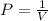
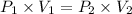
As it is seen here the volume and temperature remain constant but the volume of the container is doubled.
So, this is an example of Boyle's Law.