Answer:
For a: The examples are
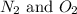
For b: The examples are
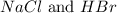
For c: The examples are
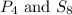
For d: The examples are
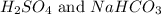
Step-by-step explanation:
Diatomic molecule is defined as the molecule which is formed by the combination of two atoms.
Polyatomic molecule is defined as the molecule which is formed by the combination of more than two atoms.
Element is defined as the chemical specie which is formed by the same kind of atoms.
For the given options:
The examples of diatomic molecules that contain atoms of the same element are
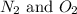
The examples of diatomic molecules that contain atoms of different element are
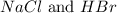
The examples of polyatomic molecules that contain atoms of the same element are
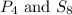
The examples of polyatomic molecules that contain atoms of different element are
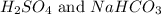
Hence, the examples are written above.